Abstract
Objectives To evaluate safety and efficacy of weekly (qw) and every other week (q2w) dosing of sarilumab, a fully human anti-interleukin 6 receptor α (anti-IL-6Rα) monoclonal antibody, for moderate-to-severe rheumatoid arthritis (RA).
Methods In this dose-ranging study, patients (n=306) with active RA, despite methotrexate, were randomly assigned to placebo or one of five subcutaneous doses/regimens of sarilumab: 100 mg q2w, 150 mg q2w, 100 mg qw, 200 mg q2w, 150 mg qw for 12 weeks, plus methotrexate. The primary end point was ACR20 at Week 12. Secondary endpoints included ACR50, ACR70, Disease Activity Score in 28 joints (C reactive protein). Safety, pharmacokinetics, pharmacodynamics and efficacy in population subgroups were assessed.
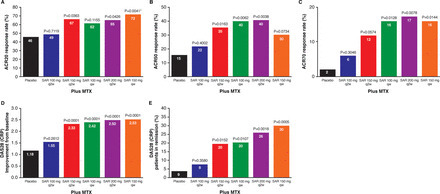
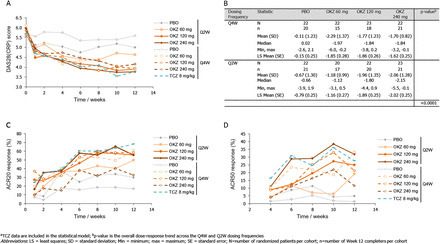
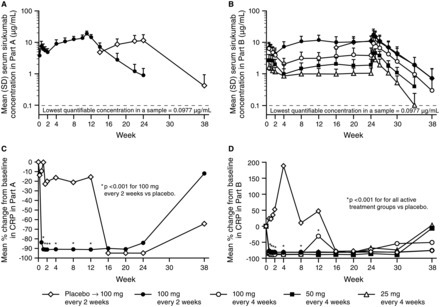
Results The proportion of patients achieving an ACR20 response compared with placebo was significantly higher for sarilumab 150 mg qw (72.0% vs 46.2%, multiplicity adjusted p=0.0203). Higher ACR20 responses were also attained with 150 mg q2w (67%; unadjusted (nominal) p=0.0363) and 200 mg q2w (65%; unadjusted p=0.0426) versus placebo. Sarilumab ≥150 mg q2w reduced C reactive protein, which did not return to baseline between dosing intervals. Infections were the most common adverse event; none were serious. Changes in laboratory values (neutropenia, transaminases and lipids) were consistent with reports with other IL-6Rα inhibitors.
Conclusions Sarilumab improved signs and symptoms of RA over 12 weeks in patients with moderate-to-severe RA with a safety profile similar to reports with other IL-6 inhibitors. Sarilumab 150 mg and sarilumab 200 mg q2w had the most favourable efficacy, safety and dosing convenience and are being further evaluated in Phase III.
Via Krishan Maggon



 Your new post is loading...
Your new post is loading...





open access full text
Ann Rheum Dis 2014;73:1626-1634 doi:10.1136/annrheumdis-2013-204405
Clinical and epidemiological researchExtended reportSarilumab, a fully human monoclonal antibody against IL-6Rα in patients with rheumatoid arthritis and an inadequate response to methotrexate: efficacy and safety results from the randomised SARIL-RA-MOBILITY Part A trialTom W J Huizinga1, Roy M Fleischmann2, Martine Jasson3, Allen R Radin4,Janet van Adelsberg4, Stefano Fiore5, Xiaohong Huang5, George D Yancopoulos4,Neil Stahl4, Mark C Genovese6+Author Affiliations
1Department of Rheumatology, Leiden University Medical Centre, Leiden, The Netherlands2Division of Rheumatology, Department of Medicine, University of Texas Southwestern Medical Center and Metroplex Clinical Research Center, Dallas, Texas, USA3Department of Research and Development, Sanofi, Paris, France4Regeneron Pharmaceuticals, Inc., Tarrytown, New York, USA5Department of Research and Development, Sanofi, Bridgewater, New Jersey, USA6Department of Immunology and Rheumatology, Stanford University Medical Center, Palo Alto, California, USACorrespondence toDr TWJ Huizinga, Chairman, Department of Rheumatology, C1-41 Leiden University Medical Center, Albinusdreef 2, PO Box 9600, Leiden 2300RC, The Netherlands;T.W.J.Huizinga@lumc.nlReceived 6 August 2013Revised 23 October 2013Accepted 24 October 2013Published Online First 2 December 2013